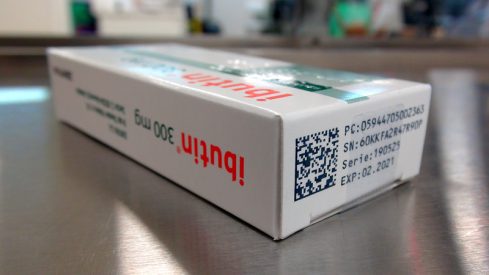
Galenica is one of the largest greek pharmaceutical companies with innovative proprietary medicines that it has already successfully exported to the Balkans. In order to comply with counterfeit drug legislation, it needed a serialization system to use for its medicines production.
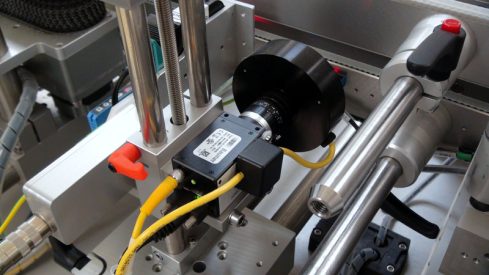
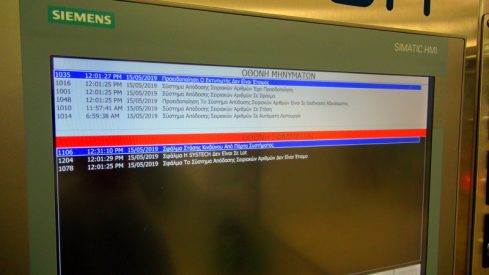
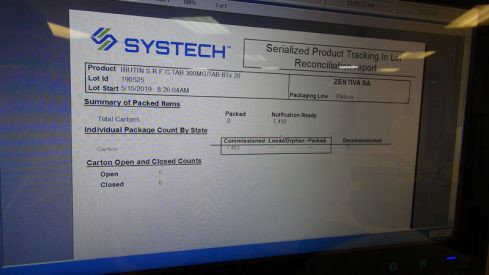
Zenon Automation SA, in response to this need, designed and implemented a complete serialization in a box system in collaboration with SYSTECH.
The Galenica pharmaceutical industry needed an integrated serialization system to monitor and detect prescription drugs throughout the supply chain, thereby achieving compliance with counterfeit drug legislation.
Serialization in a box by Zenot Automation SA: